Research Article
Diversity, Abundance and Seasonality of Ambrosia Beetles (Coleoptera: Curculionidae) in Southern Mississippi [PDF]
Werle, C. T.,1* B. J. Sampson,1 and J. B. Oliver2
1USDA-ARS, Southern Horticultural Lab, Poplarville, MS
2Tennessee State Univ., College of Agriculture, Human & Natural Sciences, Otis L. Floyd Nursery Research Ctr., McMinnville, TN
*810 Hwy 26 W, P.O. Box 287, Poplarville, MS, 39470, chris.werle@ars.usda.gov
Received: 14-VII-2011 Accepted: 7-IX-2011
Abstract: A survey was undertaken in 2010 to assess the composition of the ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) community at two research sites in southern Mississippi. Inexpensive beetle traps were constructed and fitted with ethanol lures, and bi-weekly collections were made from March through November. The granulate ambrosia beetle [Xylosandrus crassiusculus (Motschulsky)] was the most abundant xyleborine species at both sites, with more specimens collected at the Poplarville site. Other species were more abundant at the McNeill site. The black twig borer [Xylosandrus compactus (Eichhoff)] was the second-most abundant species in Mississippi, yet has not been reported from collections by collaborators in Ohio, Virginia, or Tennessee. The camphor shot borer [Xylosandrus mutilatus (Blandford)] was collected in relatively low numbers at both sites. The black stem borer [Xylosandrus germanus (Blandford)] is commonly collected by our collaborators, but none was collected from either of our research sites in southern Mississippi.
Keywords: IPM, monitoring, invasive
Introduction
Introduced populations of ambrosia beetles, mostly of Asian origin, have become a major problem for nursery growers in recent years. Adult beetles tunnel into trees and inoculate the brood gallery with symbiotic fungi, which is consumed by the larval beetles. Development is completed within the tree and emerging female beetles disperse to create new galleries (Weber and McPherson 1983). Host plants are often killed by the fungal pathogens rather than any direct beetle damage (Weber and McPherson 1984).
The granulate ambrosia beetle (GAB), Xylosandrus crassiusculus (Motschulsky), was first reported in South Carolina in 1974, and is now widely distributed across the southeastern United States. The GAB has become one of the most damaging insect pests for growers of deciduous trees, as they invest a lot of time and money on its control each spring. The camphor shot borer (CSB), Xylosandrus mutilatus (Blandford), was first detected in Starkville, MS in 1999 and is now also widely distributed across the southeast United States (Schiefer and Bright 2004). The CSB has a wide host range of tree species, similar to other ambrosia beetles (Oliver et al. 2010), but it has also been reported to attack muscadine grape vineyards (Stonea et al. 2007). The black twig borer (BTB), Xylosandrus compactus (Eichhoff), was first reported in the United States in 1941 and is now widely distributed across the Southeast (Ngoan et al. 1976). The BTB can be a serious pest of trees and shrubs, attacking the twigs and branches of drought or otherwise-stressed plants. The black stem borer (BSB), Xylosandrus germanus (Blandford), has been in North America since at least 1930 (Rabaglia et al. 2006). The BSB has become a key pest of ornamental nursery stock and is now widespread east of the Mississippi River (Ranger et al. 2010).
Population outbreaks of ambrosia beetles are difficult to detect or predict, and traditional insecticidal treatments can perform poorly. Effective chemical protection of a nursery tree crop requires treatments that are closely timed with beetle attacks, applied repeatedly, or have long residual activity. Pesticide management that is closely timed with beetle attack periods will provide the most efficacious, economical, and environmentally sound pest control. Thus, monitoring of ambrosia beetle populations is key to effective control (Hudson and Mizell 1999).
Previous studies have shown that an ethanol-baited trap can collect a variety of ambrosia beetle species, facilitating monitoring of beetle populations (Oliver et al. 2004, Ranger et al. 2010). Traps suspended within 0.5 and 1.7 m above the ground are most effective at capturing BSB and GAB, respectively, and should be placed at the lower height when monitoring for multiple ambrosia beetle species (Reding et al. 2010). Trap collection data can then be used by pest control professionals to predict emergence and to apply preventative treatments onto nursery stock.
The methodology of this ambrosia beetle monitoring research is being utilized by a team of collaborating scientists in Ohio, Virginia, Tennessee, and Mississippi. Here we discuss the populations encountered in southern Mississippi and the timing of their flights.
Materials and Methods
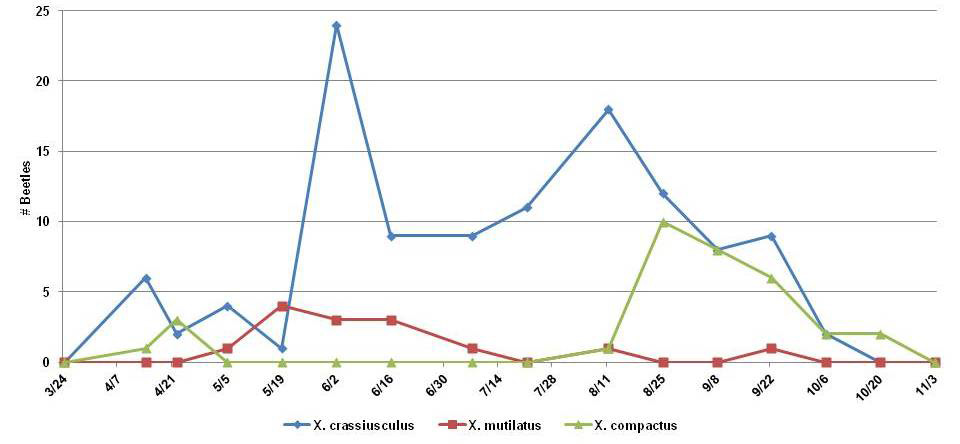
In 2010 we undertook a survey of ambrosia beetles at two sites in Pearl River County, MS (Table 1). Trapping followed the methodology and design described for using inexpensive soda bottle traps (Oliver et al. 2004, Ranger et al. 2010) baited with slow-release ethanol lures (AgBio, Westminster, CO) and suspended approximately 1 m above the ground. One site was at the Southern Horticultural Laboratory in Poplarville, MS, considered a suburban site because of its proximity to a housing subdivision and to apartments. The second site was at the Mississippi State University Experiment Station in McNeill, MS, considered a rural site because of the presence of pastures and ornamental/small fruit plantations surrounded by forest. At each site, three ethanol-baited soda bottle traps were deployed and emptied bi-weekly from March 2010 through November 2010 (Fig. 1). Insect samples were pinned and identified by using standard keys (Wood 1982, Kovach & Gorsuch 1985, Arnett et al. 2002, Dole & Cognato 2010), and were verified by using voucher specimens stored at the Otis L. Floyd Nursery Research Center, McMinnville, TN.
Results and Discussion
The most abundant xyleborine ambrosia beetle collected was GAB (70%), followed by BTB (20%) and CSB (9%) (Table 1). Unlike the other species, GAB was more abundant in Poplarville (63%), and it also had a much longer flight period (Fig. 1). Specimens were collected through September at both sites, but the peak in McNeill occurred in August, nearly two months after that of the Poplarville population.
The BTB was the second most-abundant xyleborine in our study, yet did not occur in the states of our collaborators. More abundant in McNeill (64%), it was also notable that BTB outbreaks were fairly synchronized between the two sites. The largest BTB collection occurred in August–September, with a much smaller collection spike in April at both sites.
The CSB was least abundant of the three xyleborine species, with only 14 specimens collected. Numbers of CSB peaked at both sites in May–June, and it was more abundant in McNeill (64%). The abundance of CSB at McNeill is a concern as this research site is home to a large experimental plantation of muscadine grapes. Meticulous scouting of this vineyard will be necessary to prevent establishment of CSB.
Table 1. Species diversity and abundance of ambrosia beetles in southern Mississippi.
Species |
Abundance |
|
McNeill, MS |
Poplarville, MS |
|
Xylosandrus crassiusculus |
42 |
73 |
Xylosandrus mutilatus |
9 |
5 |
Xylosandrus compactus |
21 |
12 |
Xyleborinus saxeseni |
21 |
9 |
Xyleborus affinis |
1 |
2 |
Hypothenemus dissimilis |
301 |
112 |
Ambrosiodmus lecontei |
- |
1 |
Monarthrum mali |
1 |
- |
Micracisella nanula |
3 |
1 |
Hylocurus rudis |
1 |
- |
Euplatypus compositus |
1 |
- |
Figure 1. Seasonality of three species of Xylosandrus ambrosia beetles in southern Mississippi in 2010.
The BSB was not collected at either of our research sites. It is interesting to note that this beetle is a dominant species in Ohio, slightly less so in Virginia, rare in Tennessee, and completely absent from our collections in southern Mississippi. It is possible a longitudinal gradient effect occurs among the ambrosia beetles, with BSB becoming more abundant to the north and BTB more abundant to the south. Future research is planned to test this hypothesis.
Other Scolytinae ambrosia beetles collected in our traps included the cryphaline Hypothenemus dissimilis Zimmermann, the corthyline Monarthrum mali (Fitch), the micracines Micracisella nanula (LeConte) and Hylocurus rudis (LeConte), and the xyleborines Xyleborinus saxeseni (Ratzeburg), Xyleborus affinis Eichhoff, and Ambrosiodmus lecontei Hopkins. We also collected the platypodine ambrosia beetle Euplatypus compositus (Say). Other non-ambrosia beetles of note included the hylesinine Cnesinus strigicollis LeConte and the bostrichid Xylobiops basilaris (Say), both of which were collected in high numbers throughout the sample period.
In addition to these xylophilus species, we discovered some beetles that are potentially of use as biocontrol agents. The clerids Neorthopleura thoracica (Say), Phyllobaenus verticalis (Say), and Cregya mixta LeConte, and the anthicid Anthicus cervinus Laferte, were all collected from our traps.
Acknowledgments
We thank Alicia Bray and Nadeer Youssef, both of Tennessee State University’s Otis L. Floyd Nursery Research Center in McMinnville, TN, for insect identification training/verification and for instruction on beetle trap construction, respectively.
References
Arnett, Jr., R. H., M. C. Thomas, P. E. Skelley, and J. H. Frank. 2002. American beetles. CRC Press, Boca Raton, FL. 1304 pp.
Batra, L. R. 1967. Ambrosia fungi: a taxonomic revision and nutritional studies of some species. Mycologia 59: 976-1017.
Dole, S. A. and A. I. Cognato. 2010. Phylogenetic revision of Xylosandrus Reitter. Proc. Calif. Acad. Sci. 61: 451-545.
Hudson, W. and R. Mizell. 1999. Management of an Asian ambrosia beetle, Xylosandrus crassiusculus, in nurseries. Proc. South. Nursery Assn. Res. Conf. 44: 198-201.
Kovach, J. and S. S. Gorsuch. 1985. Survey of ambrosia beetle species infesting South Carolina peach orchards and a taxonomic key for the most common species. J. Agric. Entomol. 2: 238-247.
Ngoan, N. D., R. C. Wilkinson, D. E. Short, and C. S. Moses. 1976. Biology of an introduced ambrosia beetle, Xylosandrus compactus. Ann. Ent. Soc. Am. 69: 872-876.
Oliver, J. B., N. N. Youssef, and M. A. Halcomb. 2004. Comparison of different trap types for collection of Asian ambrosia beetle, pp. 158-163. In J.B. Oliver [ed.], Proc. 49th Ann. South. Nursery Assoc. Res. Conf. 11-12 Aug. 2004. Atlanta, GA.
Oliver, J., N. Youssef, J. Basham, K. Copley, M. Halcomb, F. Hale, and W. Haun. 2010. The camphor shot borer Tennessee’s new invasive ambrosia beetle. Tenn. Green Times. 11: 8-11.
Rabaglia, R. J., S. A. Dole, and A. I. Cognato. 2006. Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occurring North of Mexico, with an illustrated key. Ann. Entomol. Soc. Am. 99: 1034-1055.
Ranger, C. M., M. E. Reding, A. B. Persad, and D. A. Herms. 2010. Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles. J. Agric. For. Entomol. 12: 177-185.
Reding, M. E., J. Oliver, P. Schultz, and C. M. Ranger. 2010. Monitoring flight activity of ambrosia beetles in ornamental nurseries with ethanol-baited traps: influence of trap height on captures. J. Environ. Hortic. 28: 85-90.
Schiefer, T. L. and D. E. Bright. 2004. Xylosandrus mutilatus (Blandford), an exotic ambrosia beetle (Coleoptera: Scolytinae: Xyleborini) new to North America. Coleop. Bull. 58: 431-438.
Stonea, W. D., T. E. Nebeker, and P. D. Gerard. 2007. Host plants of Xylosandrus mutilatus in Mississippi. Fla. Entomol. 90: 191-195.
Weber, B. C. and J. E. McPherson. 1983. Life history of the ambrosia beetle Xylosandrus germanus (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 76: 455-462.
Weber, B. C. and J. E. McPherson. 1984. The ambrosia fungus of Xylosandrus germanus (Coleoptera: Scolytidae). Can. Entomol. 116: 281-283.
Wood, S. L. 1982. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat. 6: 1359 pp.