Research Article
Comparison of Cotton Damage from Tarnished Plant Bug (Hemiptera: Miridae) and Southern Green Stink Bug (Hemiptera: Pentatomidae) Adults and Nymphs [pdf]
Musser, F. R.*, K. S. Knighten, and J. T. Reed
Department of Entomology & Plant Pathology, Mississippi State University, Mississippi State, MS 39762
* Corresponding author: Dept. of Entomology and Plant Pathology, Mississippi State University, P. O. Box 9775, Mississippi State, MS 39762 fm61@msstate.edu
Received: 21-VII-2008 Accepted: 25-VIII-2008
Keywords: Gossypium hirsutum, Lygus lineolaris, Nezara viridula, threshold, boll damage
Introduction
Tarnished plant bug, Lygus lineolaris (Palisot de Beauvois) (Hemiptera: Miridae) and stink bugs (a complex largely comprised of southern green stink bug, Nezara viridula (L.), green stink bug, Acrosternum hilare (Say) and brown stink bug, Euschistus servus (Say) (Hemiptera: Pentatomidae)) are the primary targets for a large portion of the insecticides being applied to cotton (Gosspyium hirsutum L.) in the Midsouth (Williams 2008). Cotton damage from tarnished plant bugs is primarily from feeding on cotton squares (flower buds) (Tugwell et al. 1976) while stink bugs cause economic damage to cotton by feeding on medium-aged bolls (fruiting structures) (Siebert et al. 2005). However, plant bugs and stink bugs are frequently observed concurrently in a cotton field and the source of damage on a square or boll cannot be distinguished for these bugs (Greene et al. 1999). Feeding by plant bugs and stink bugs is accompanied by the injection of saliva into the plant that disrupts plant development. In young squares feeding generally results in square abscission, while feeding on larger squares often results in damaged anthers (Pack and Tugwell 1976). When anther damage exceeds 30%, pollination is affected, which can cause bolls to abscise or be malformed (Tugwell et al. 1976). Feeding damage to young bolls may cause abscision, but more typically results in external dark lesions, internal growths on the carpal wall, stained lint, or undeveloped locules within the boll (Layton 2000). Once the boll has accumulated 300 heat units after anthesis (about 12 days), tarnished plant bugs are no longer able to damage the boll (Horn et al. 1999, Emfinger et al. 2004). However, southern green stink bugs can still damage the boll until it is about 18 days old (Greene et al. 1999) or has accumulated 500 heat units (Bommireddy et al. 2007).
While the symptoms of feeding of plant bugs and stink bugs are similar, the amount of damage may not be similar. Furthermore, the choice of feeding sites may vary between species and between adults and nymphs. In two previous studies (Greene et al. 1999, Bommireddy et al. 2007), stink bugs and/or tarnished plant bugs have been caged on individual fruiting structures to evaluate damage. However, these no-choice assays may be overestimating damage because the insect may not choose to feed on that structure when other food sources are available. To overcome this limitation, the current study caged insects on a whole plant so the insects could select their feeding site. Therefore, the objective of this research was to compare the type and severity of damage to cotton squares and bolls from various densities of tarnished plant bug and southern green stink bug adults and nymphs. It is expected that findings from this study will facilitate development of a pest management system that manages these species in a sustainable manner while minimizing economic losses.
Materials and Methods
This trial was conducted at the R. R. Foil Plant Science Research Facility at Mississippi State University in Mississippi State, MS during 2004, 2005 and 2006. A Bollgard or Bollgard II cotton variety was grown by using typical cotton production practices, excluding insecticide use, on 97-cm rows each year. Insects were caged on cotton in single plant sleeve cages when the cotton had numerous bolls but was still producing a few squares. The sleeve cages were made of 1 mm mesh tulle, and were 32 cm in diameter and 100 cm long. They were closed around the plant at least 5 nodes below the flowering node using a ponytail holder, infested with an insect treatment, and then closed at the top of the plant with a second ponytail holder. Southern green stink bugs were collected from soybean fields within a few days of infestation and were maintained on cotton bolls and soybean pods until used in the trial, while most tarnished plant bugs were from a laboratory colony maintained on an artificial diet (Cohen 2000). Insects were kept in the sleeve cages for 7, 4 and 4 days in 2004, 2005 and 2006, respectively. Cages were then removed and the plants were sprayed with an insecticide to remove all natural and introduced bugs from the field. After the bolls opened, the plants were cut and harvest data (aborted bolls, lint and seed weight, number of stained or hard locules, number of seeds and carpel damage) were collected by fruiting position on the plant. In 2004, cages were infested with 0, 1, 2, 5 or 10 southern green stink bug adults or 4th–5th-instar nymphs between August 8 and August 13. In 2005 and 2006, infestations of tarnished plant bug and southern green stink bug adults and 4th–5th-instar nymphs were made with densities of 0, 1, 2, 4 or 8 insects per cage between August 8 and August 25, 2005, and between July 14 and August 11, 2006. The entire plant was evaluated for damage in 2004, but in 2005 and 2006, the node with a first-position white flower was identified and marked with a label at the time of infestation. After the bolls opened, first-position bolls three nodes above to five nodes below the flowering node at the time of infestation were examined. After harvest in 2005 and 2006, the cotton was ginned and at least two samples from each treatment (replicates were combined as needed to have enough cotton for evaluation) were analyzed by using High Volume Instrument classification (Ramey 1995) by the USDA cotton classing office in Memphis, TN. All harvest data were divided into ‘bottom’ and ‘top’ portions. The bottom portion included first-position bolls located one to five nodes below the white flower at the time of infestation. The top portion contained those first-position bolls located at nodes from the white flower to three nodes above the white flower at the time of infestation. Undamaged bolls were used as a measure of insect impact because it was a composite measure that accounted for fruit abscission as well as damaged fruit that remained on the plant until harvest.
Data Analysis. Data were analyzed by using PROC MIXED in SAS Version 9.1 (SAS Institute 1999). In 2004, infestation date was treated as a random variable and individual plants were the experimental unit. There were 24 replicates of stink bug adult infestation rates, 7 replicates of stink bug nymph infestation rates and 31 replicates of the uninfested control. To compare yields at various nodes on the plant, bolls from the second and third positions were grouped with the first-position bolls of a similar maturity. For example, data from a second-position boll at the seventh node was recorded with a first-position boll at the ninth node because they would have flowered on the same day (Univ. CA 1996). Because sample sizes were relatively small in 2004, data from two adjacent nodes were combined for statistical analysis. The uppermost node combination includes bolls from three nodes (15, 16 and 17).
In 2005 and 2006, the trials were designed as a randomized complete block with 4 and 5 replicates in 2005 and 2006, respectively, and blocked over time. Five sleeve cages were used as the experimental unit. Because methods were identical in 2005 and 2006 and because the results were similar, data from both years were analyzed together. To distinguish between the damage caused by each insect type, densities of 4 and 8 insects per cage were compared to the untreated control by using a χ2 test at each node for the numbers of fruit aborted, damaged or undamaged at harvest. Yield and cotton quality parameters were analyzed with PROC MIXED. Data were transformed by square root as needed to stabilize variance and meet the assumptions of normally distributed data. The nine infestation dates (replications) were treated as a random factor. When interactions were not significant (α = 0.05), they were removed from the model and the data were analyzed again.
Results
2004 Southern Green Stink Bug Trial. Higher adult densities corresponded with reduced overall lint yield (F = 9.37, df = 4,121, P < 0.0001), but nymphs caused no significant yield losses (F = 1.46, df = 4,54, P = 0.2263) (Table 1). The proportion of locules damaged by adults also increased with increasing stink bug density (F = 16.82, df = 4,117, P < 0.0001). While nymphs had no impact on yield, the proportion of damaged locules did increase in some of the nymph-infested treatments (F = 3.59, df = 4,53, P = 0.0115) (Table 1). Examination of the yield data by boll position shows that the yield losses from adults occurred on each node grouping from node 7/8 to 11/12, but no nodes were impacted by nymphs (Fig. 1) Differences between treatments were also significant for node 5/6, with yields of the two highest densities significantly higher than the control. There were significant differences among nymph treatments on nodes 13/14 and 15–17, but these were actually increased yields for 1 and 10 nymph densities compared to the uninfested control. The first position flowering node at the time of infestation was generally between 13 and 16 in 2004, so the nodes impacted by adults were small to medium sized bolls at the time of infestation. The proportion of damaged locules by node position shows similar results to the yield data with significant losses from stink bugs on nodes 5–12 (Fig. 2). The proportion of damaged locules by nymphs was significantly greater at nodes 9–10 even though a yield loss was not measured (Fig. 2B). The small sample size likely prevented locule damage at other nodes from being significantly different from the control. Bolls on nodes 13–17, which were squares or flowers at the time of infestation, were not impacted by stink bug adults of nymphs.
Table 1. Overall least squared mean yield (± SEM) and proportion of locules (± SEM) damaged by southern green stink bug adults and nymphs in 2004. Treatments followed with the same letter within a column are not significantly different (P = 0.05). Overall F-test for nymph yield not significant (F = 1.46, df = 4,54, P = 0.226).
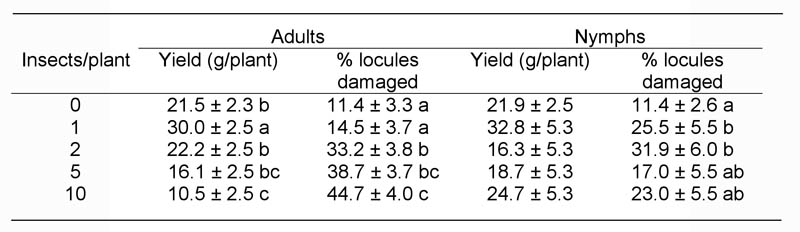
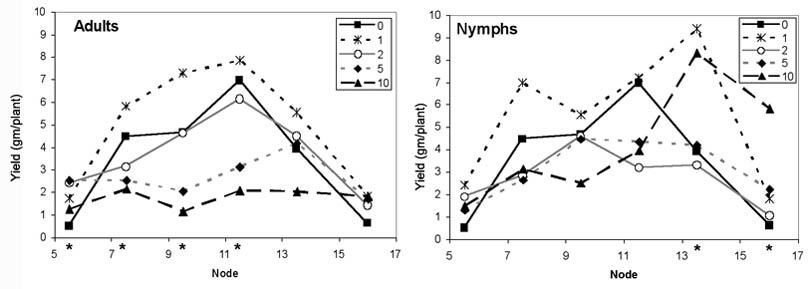
Figure 1. Cotton yield (gm per plant) in 2004 when infested with southern green stink bug adults or nymphs during August. Control data are the same in both graphs. Data from two adjacent nodes were combined for analysis. Asterisks denote nodes where yield was significantly different among different stink bug densities (P = 0.05).
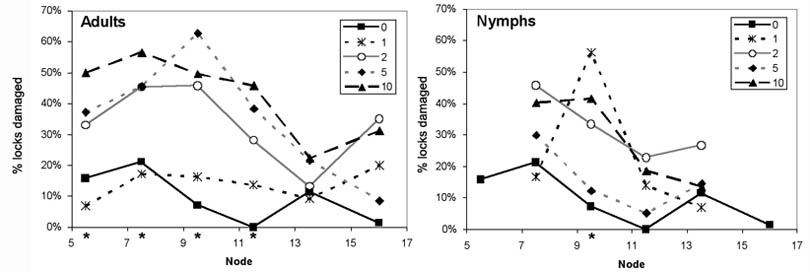
Figure 2. Proportion of locules damaged in 2004 when infested with southern green stink bug adults or nymphs during August. Control data are the same in both graphs. Data for lowest and highest nodes not shown for nymphs as data were drawn from too few bolls to provide meaningful data. Data from two adjacent nodes (three nodes for 15–17) combined for analysis. Asterisks denote nodes where proportion damaged was significantly different among different stink bug densities (P = 0.05).
2005 and 2006 Stink Bug and Plant Bug Trial. The first-position flowering node was generally between 10 and 16 at the time of infestation. Each of the five nodes immediately below the infested white flower had significantly fewer undamaged bolls when infested with southern green stink bugs, but the tarnished plant bug-infested cages had a similar percent of undamaged bolls as the uninfested control (Fig. 3). No other node showed a significant impact on percent undamaged bolls from the insect types. The total number of undamaged locules on the five nodes below the infested flower node were significantly impacted by the insect type (F = 4.19, df = 4,133, P = 0.0032) and density (F = 19.95, df = 1,133, P < 0.0001) caged on the plant (Fig. 4A). The interaction of insect and number released was also significant (F = 6.32, df = 3,133, P = 0.0005) as more stink bugs decreased the number of undamaged locules at harvest, but tarnished plant bugs had no impact. In the nodes above the flower, insect type (F = 1.59, df = 4,134, P = 0.1803), number released (F = 0.71, df = 1,134, P = 0.4008) and their interaction (F = 1.05, df = 3,134, P = 0.3706) were not significant factors (Fig. 4B).
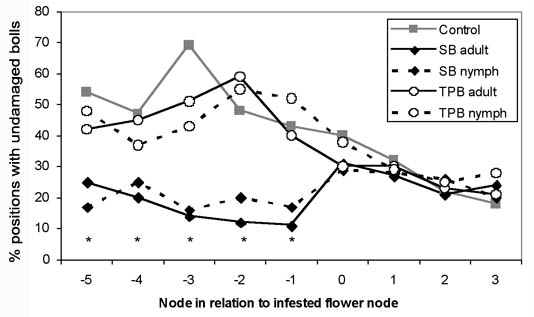
Figure 3. Percent of first-position sites with an undamaged boll at harvest in 2005 and 2006 by node in relation to the flower node when uninfested (control) or infested with tarnished plant bug (TPB) adults or nymphs, or southern green stink bug (SB) adults or nymphs. Asterisk above a node indicates that the χ2 test was significant at that node (P = 0.05). Only insect densities of 0, 4 or 8 insects per cage were used in this analysis.
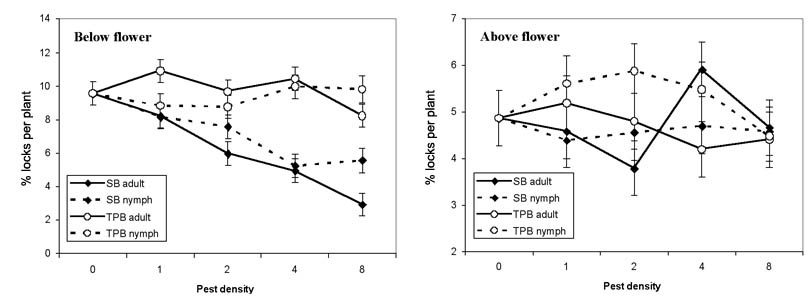
Figure 4. Number of undamaged locules present (± SEM) from first-position bolls at 1 to 5 nodes below the infested white flower and infested flowering node to 3 nodes above infested flower node, when infested with various densities of southern green stink bugs (SB) or tarnished plant bugs (TPB)
Yields from first-position bolls above and below the white flower at the time of infestation were impacted by southern green stink bugs in a similar manner as the number of undamaged bolls (Fig. 5). Adults and nymphs reduced the yield from bolls below flower (adult t = -6.38, df = 138, P < 0.0001; nymph t = -4.45, df = 138, P < 0.0001), but had no significant impact on yield from the nodes that were squares during infestation (adult t = 0.71, df = 138, P = 0.4815; nymph t = 0.43, df = 138, P = 0.6712). Tarnished plant bugs had little effect on yield above or below the flowering node (adult above t = -1.19, df = 138, P = 0.2348; nymph above t = 0.20, df = 138, P = 0.8397; adult below t = 0.19, df = 138, P = 0.8502; nymph below t = 1.11, df = 138, P = 0.2668).
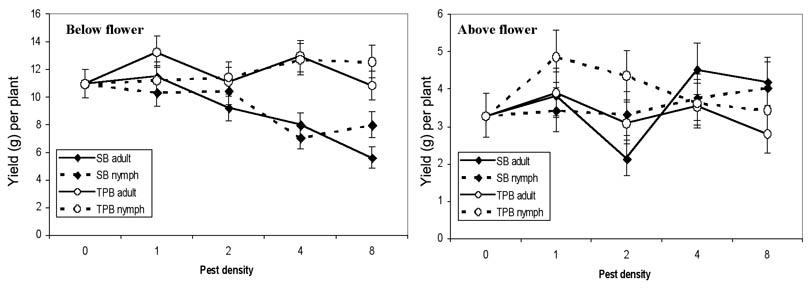
Figure 5. Seed cotton yield (± SEM) (back-transformed grams per plant) from first-position bolls at 1 to 5 nodes below the infested white flower and infested flowering node to 3 nodes above infested flower node, when infested with various densities of southern green stink bugs (SB) or tarnished plant bugs (TPB).
In addition to yield differences among treatments, there were also lint quality differences due to insect feeding. Micronaire, uniformity, yellowness and grayness had significant differences among treatments on bolls below the infested white flower (Fig. 6). Micronaire decreased as insect density increased (F = 12.29, df = 1,77, P = 0.0008). However, there was not a significant difference among insect species and life stage (F = 0.40, df = 4,77, P = 0.8106). There was a significant interaction of insect species and insect density on lint uniformity (F = 3.75, df = 3,74, P = 0.0145), largely due to the increased uniformity in the tarnished plant bug nymph treatment at high insect densities. Differences in grayness between insect species and stage were nearly significant (F = 2.43, df = 4,77, P = 0.0548), but insect density was not a significant factor (F = 2.24, df = 1,77, P = 0.1384). Yellowness increased as stink bug adult density increased, so both insect species and stage (F = 3.26, df = 4,77, P = 0.0160) and density (F = 10.26, df = 1,77, P = 0.0020) were significant factors for this lint quality. Lint length, strength and trash from bolls below the infested flower node were not impacted by insect species, stage or density (data not shown). No quality parameters from the bolls above the infested node were significantly affected by insect species, stage or density (data not shown).
Discussion
Southern green stink bug adults and nymphs caused more damage to young bolls than tarnished plant bugs in both 2005 and 2006. This damage was reflected both in yield and in quality losses. Therefore, the lower thresholds currently recommended for stink bugs (e.g. Catchot 2008) are justified by these data. Stink bug adults tended to cause more damage than late-instar stink bugs in all 3 years of the study. This finding is in contrast to Greene et al. (1999), who found fifth instars caused more damage than adults, and Bommireddy et al. (2007), who found no differences between adults and nymphs. These differences may be explained by differences in methodology. While Greene et al. and Bommireddy et al. caged stink bugs with a single boll, we caged southern green stink bugs on a whole plant. Nymphs may feed on plant parts other than bolls when given the choice, which could explain the difference in results. Regardless of whether late instars cause slightly more or less damage, late instars soon become adults. Furthermore, the differences in damage were not great enough in these studies to merit separate thresholds for adults and nymphs. In our studies, the boll on the fifth node below the flower had accumulated 300–400 heat units since anthesis (Bagwell and Tugwell 1992), which is still within the 500 heat unit vulnerable period for stink bug damage (Siebert et al. 2005, Bommireddy et al. 2007). Therefore our finding of stink bug damage to bolls five nodes below the flowering node is consistent with previous research. All fiber quality components have been previously reported to be impacted by stink bug feeding (Bommireddy et al. 2007). In this study, stink bugs impacted micronaire, uniformity and color, but we did not observe an impact on fiber length or strength. This difference may be a result of feeding intensity. In the Bommireddy et al. (2007) study, one southern green stink bug was infested on a single boll, but in our study a maximum of eight southern green stink bugs were infested on a plant that typically contained at least 5 bolls plus several squares, leaves and stems. As a result, the stink bug damage intensity in our study may not have been as severe, so impacts on fiber length and strength were not observed.
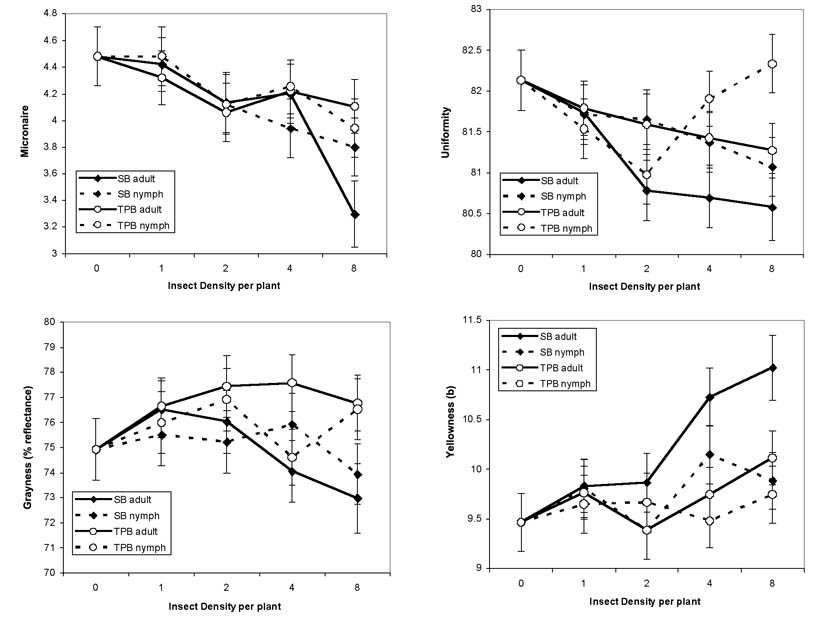
Figure 6. HVI cotton fiber quality as measured for micronaire, uniformity, grayness and yellowness from first-position bolls located 5 nodes below the infested white flower when infested with various densities of southern green stink bugs (SB) or tarnished plant bugs (TPB).
This experiment was conducted late in the flowering period and plants were unable to fully compensate for lost bolls as has been observed with early-season insect infestations (Jubb and Carruth 1971, Gaylor et al. 1983, Holman and Oosterhuis 1999, Stewart et al. 2001). However, there was still a trend of higher yields above the infested flower on treatments with the lowest yield below the infested flower (Fig. 3) and higher yields on the very lowest nodes on infested treatments (Fig. 1). The reduced ability of plants to compensate means that yield losses from late season damage will more consistently reduce yields than earlier-season feeding as described by Willrich et al. (2004). Therefore lower thresholds may be economically beneficial at the end of the flowering period rather than earlier in the season. Furthermore, at this time most of the bolls are still vulnerable to stink bug damage (Fig. 2).
While tarnished plant bugs are recognized as serious pests during the flowering period that can reduce yields of cotton (Gore and Catchot 2005, Greene et al. 2007), they failed to significantly damage cotton during the late flowering period in this experiment. Results of this experiment may have been different earlier in the flowering period. Another factor that may have reduced the impact of tarnished plant bugs in this experiment was survival in the cages. At the completion of the 4-day trial, plants were examined carefully to recover all the insects. Live stink bugs were recovered at a much higher rate (74 and 51% for adults and nymphs, respectively) than tarnished plant bugs (46 and 24% for adults and nymphs, respectively). This was partly due to higher mortality rates in tarnished plant bugs, but the smaller size and more cryptic coloration of tarnished plant bugs, especially the nymphs, was also a factor that reduced the recovery rate. No correction was made for varying recovery rates since it was not known when the insects died, or how many were still feeding but undetected. Another factor that may have played a role in the difference between southern green stink bugs and tarnished plant bugs was the insect source. Stink bugs were recently collected from soybeans and kept on cotton bolls and soybean pods until they were used in the experiment. Tarnished plant bugs came from a one-year-old laboratory colony fed on an artificial diet. While some of the plant bugs survived on the cotton plants, they may not have fed as aggressively as would wild tarnished plant bugs. A third factor that varied between the southern green stink bugs and tarnished plant bugs was their behavior on the cotton plant. When removing the cages from the cotton plants, we frequently observed plant bugs on the cage material, while stink bugs were generally found on the cotton plant itself. Cotton is known to be a lesser-preferred host for tarnished plant bugs (Ferreira 1979, Hatfield et al. 1983, Fleischer and Gaylor 1988). Therefore plant bugs may have fed minimally on the cotton because they were not accustomed to this diet. There is some evidence that populations of tarnished plant bugs in areas that have a large proportion of the land in cotton have become more adapted to cotton (G. Snodgrass, unpublished data). If this experiment had been conducted with such a population, the results may have differed.
Southern green stink bugs and tarnished plant bugs are found concurrently in cotton, and cause similar damage symptoms. However, based on this study, cotton near the end of the flowering period is much more vulnerable to stink bug feeding than plant bug feeding. The lowest stink bug densities in this trial caused damage. Because this density exceeded the currently recommended thresholds (Catchot 2008), the results of this study support the current stink bug threshold. However, the current study could not measure significant damage from tarnished plant bugs at any density, so further research is needed to determine if tarnished plant bug thresholds should be raised during the late flowering period.
Acknowledgements
The authors thank two anonymous reviewers, as well as J. Gore, K. C. Allen, and J. F. Smith for reviewing earlier versions of this manuscript. We also thank numerous summer workers for their assistance in data collection. This article was approved for publication as journal article J-11409 of the Mississippi Agricultural and Forestry Experiment Station, Mississippi State University. Partial funding was provided by the Cotton Incorporated Core Program.
References Cited
Bagwell, R. D., and N. P. Tugwell. 1992. Defining the period of boll susceptibility to insect damage in heat-units from flower. pp. 767-768. In: Proceedings, Beltwide Cotton Conferences. National Cotton Council, Memphis, TN.
Bommireddy, P. L., B. R. Leonard, and J. H. Temple. 2007. Influence of Nezara viridula feeding on cotton yield, fiber quality, and seed germination. J. Econ. Entomol. 100: 1560-1568.
Catchot, A. 2008. Insect control guide for corn, cotton, & soybeans 2008. Mississippi State University Extension Service, Publication 2471.
Cohen, A. C. 2000. New oligidic production diet for Lygus hesperus Knight and L. lineolaris (Palisot de Beauvois). J. Entomol. Sci. 35: 301-310.
Emfinger, K. D., B. R. Leonard, M. M. Willrich, J. D. Siebert, J. H. Fife, J. S. Russell, J. Gore, and J. J. Adamczyk. 2004. Defining boll and yield tolerance to late-season cotton insect pests in Louisiana. pp. 278-281. In: Proceedings, Beltwide Cotton Conferences. National Cotton Council, Memphis, TN.
Ferreira, J. M. S. 1979. Ethology of host plant feeding preference by the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois). M.Sc. thesis, Mississippi State University, Mississippi State, MS.
Fleischer, S. J., and M. J. Gaylor. 1988. Lygus lineolaris (Heteroptera: Miridae) population dynamics, nymphal development, life tables and Leslie matrices on selected weeds and cotton. Environ. Entomol. 17: 246-253.
Gaylor, M. J., G. A. Buchanan, F. R. Gilliland, and R. L. Davis. 1983. Interactions among a herbicide program, nitrogen fertilization, tarnished plant bugs (Lygus lineolaris) and planting dates for yield and maturity of cotton, Gossypium hirsutum. Agron. J. 75: 903-907.
Gore, J., and A. Catchot. 2005. Tarnished plant bug sampling and management in the Mississippi Delta. pp. 1234-1238. In: Proceedings, Beltwide Cotton Conferences. National Cotton Council, Memphis, TN.
Greene, J. K., S. G. Turnipseed, M. J. Sullivan, and G. A. Herzog. 1999. Boll damage by southern green stink bug (Hemiptera: Pentatomidae) and tarnished plant bug (Hemiptera: Miridae) caged on transgenic Bacillus thuringiensis cotton. J. Econ. Entomol. 92: 941-944.
Greene, J. K., C. Milligan, C. Capps, G. M. Lorenz, K. Colwell, and G. E. Studebaker. 2007. Management considerations for the sucking bug complex - 2005. pp. 436-447. In: Proceedings, Beltwide Cotton Conferences, Memphis, TN.
Hatfield, L. D., J. Ferreira, and D. L. Frazier. 1983. Host selection and feeding behavior by the tarnished plant bug, Lygus lineolaris (Hemiptera: Heteroptera: Miridae). Ann. Entomol. Soc. Am. 76: 688-691.
Holman, E. M., and D. M. Oosterhuis. 1999. Cotton photosynthesis and carbon partitioning in response to floral bud loss due to insect damage. Crop Sci. 39: 1347-1351.
Horn, T. O., F. A. Harris, J. T. Robbins, and R. E. Furr, Jr. 1999. Influence of boll age on susceptibility to tarnished plant bug injury. pp. 1044-1045. In: Proceedings, Beltwide Cotton Conferences. National Cotton Council, Memphis, TN.
Jubb, G. L., and L. A. Carruth. 1971. Growth and yield of caged cotton plants infested with nymphs and adults of Lygus hesperus. J. Econ. Entomol. 64: 1229-1236.
Layton, M. B. 2000. Biology and damage of the tarnished plant bug, Lygus lineolaris, in cotton. Southwest. Entomol. Suppl. 23: 7-20.
Pack, T. M., and P. Tugwell. 1976. Clouded and tarnished plant bugs on cotton: A comparison of injury symptoms and damage of fruit parts. Arkansas Agricultural Experiment Station, Report Series 226.
Ramey, H. H., Jr. 1995. Universal standardization of HVI measurements. pp. 1173-1175. In: Proceedings, Beltwide Cotton Conferences. National Cotton Council of America, Memphis, TN.
SAS Institute. 1999. SAS for Windows, Version 9.1. SAS Institute, Inc., Cary, NC, USA.
Siebert, M. W., B. R. Leonard, R. H. Gable, and L. R. LaMotte. 2005. Cotton boll age influences feeding preference by brown stink bug (Heteroptera: Pentatomidae). J. Econ. Entomol. 98: 82-87.
Stewart, S. D., M. B. Layton, M. R. Williams, D. Ingram, and W. Maily. 2001. Response of cotton to prebloom square loss. J. Econ. Entomol. 94: 388-396.
Tugwell, P., S. C. Young, B. A. Dumas, and J. R. Phillips. 1976. Plant bugs in cotton: importance of infestation time, types of cotton injury, and significance of wild hosts near cotton. University of Arkansas Agricultural Experiment Station, Report Series 227.
Univ. CA. 1996. Integrated Pest Management for Cotton in the Western Region of the United States. University of California, Division of Agriculture and Natural Resources, Publication 3305.
Williams, M. R. 2008. Cotton insect losses, http://www.entomology.msstate.edu/resources/tips/cotton-losses/data/.
Willrich, M. M., B. R. Leonard, R. H. Gable, and L. R. LaMotte. 2004. Boll injury and yield losses in cotton associated with brown stink bug (Heteroptera: Pentatomidae) during flowering. J. Econ. Entomol. 97: 1928-1934.
