Research Article
Effects of Temperature on the Metabolic Rates of Insecticide Resistant and Susceptible German Cockroaches, Blattella germanica (L.) (Dictyoptera: Blattellidae) [pdf]
Dingha, B. N.1*, A. G. Appel1, J. T. Vogt2
1Department of Entomology and Plant Pathology, Auburn University, AL
2USDA-ARS, NBCL, Stoneville, MS
*Current Address: George Washington Carver Agricultural Experiment Station & Department of Agriculture and Environmental Sciences, Milbank Hall, Rm 204B, Tuskegee, AL 36088. nuck40@yahoo.com
Received: 5-XI-2008 Accepted: 20-I-2009
Abstract We examined the standard metabolic rates of pyrethroid resistant and susceptible German cockroaches, B. germanica. Measurements of CO2 production and O2 consumptions were obtained at 5 to 40 °C at 5 °C intervals using closed-system respirometry. Overall VO2 (ml g-1 h-1) was not significantly different among strains but different strains reacted differently to increasing temperature. Mean cockroach body mass differed significantly among strains and VO2 (ml g-1 h-1) scaled with temperature and mass in all strains. We used data at 25 °C to examine the effect of mass on the rate of oxygen consumption. Mass specific VO2 (ml g--1 h-1) at 25 °C was 0.5170 ± 0.13 and 0.6931 ± 0.059 for the susceptible strains (black and ACY, respectively) and 0.7714 ± 0.059 for the resistant strain. The relationship between metabolic rate and body mass yielded an exponent of 0.78 for the resistant strain and 0.81 and 0.89 for the susceptible strains (black and ACY, respectively), and are similar to those of other cockroaches. Measurement of O2 and CO2 made possible the examination of temperature and mass effects on respiratory quotient (RQ). Respiratory quotient increased with temperature up to about 25 °C, declining thereafter in all the strains; however there were differences in response among strains. Several equations relating temperature and mass to VO2 are presented. The supercooling point of the resistant strain was significantly greater than those of the ACY and black susceptible strains.
Key words: standard metabolic rate, German cockroach, permethrin resistance, respiratory quotient
Introduction
Temperature is the most important determinant of metabolic rate in ectothermic animals (Cossins and Bowler, 1987; Hawkins 1995; Angilletta et al. 2002; Gillooly et al. 2001) and controls nearly all physiological and biochemical processes (Huey and Berrigan, 2001). Increases in temperature generally result in increases in physiological processes including metabolic rate. Metabolic rate in arthropods also varies as a consequence of locomotion (Rogowitz and Chappell, 2000), gender (Rogowitz and Chappell, 2000), altitude (Rourke, 2000), parasitism (Kolluru et al. 2002), water scarcity (Davis et al. 1999), climate (Nielsen et al. 1999), body mass (Gillooly et al. 2001), reproduction (Prestwich and Walker, 1981), and in the presence of insecticides and heavy metals (Kramarz and Kafel, 2003; Dingha et al. 2004).
Metabolic rate is a measure of the energetic cost of living, which in turn exerts a major influence on the fitness of organisms. Knowledge of the metabolic rate of an organism would provide an insight into its energetic costs, thereby revealing patterns of energy use. To determine if there is an energetic cost associated with physiological resistance to insecticide, we measured the rates of O2 consumption and CO2 production in the German cockroach, Blattella germanica (L).
B. germanica is a world-wide household pest that may harbor and transmit human disease-causing pathogens (Ramirez, 1989). Their body parts and feces are also potent allergens to sensitive people (Roberts, 1996). Pyrethroid insecticides are widely used for cockroach control because of their effectiveness and low mammalian toxicity. However, control failures in some field populations have been reported as a result of the development of resistance (Cochran, 1989; Valles et al. 2000). It has been reported that resistance levels generally decline in the absence of insecticide selection (Tabashnik et al.1994; Rahardja and Whalon, 1995), and that decreased resistance is associated with increased fitness (Tabashnik et al. 1994). Therefore, we hypothesized that genetically resistant B. germanica not exposed to insecticides for several generations would have similar metabolic rates and respond to temperature change as would a susceptible strain. We also described the effect of temperature on metabolic processes using Q10, which is defined as the change in the rate of metabolism over 10 °C change in temperature. In addition, we obtained respiratory quotients (RQ) and mass scaling relationships for each strain and compared these with other cockroaches.
Materials and Methods
Three strains of B. germanica were used in this study. A black body mutant (Ross and Cochran, 1975) (black), and ACY (American Cyanamid Clifton, NY) were the susceptible strains. Both strains have been reared in the laboratory without exposure to insecticide for > 40 years. The insecticide resistant strain (Apyr-R) was collected from infested apartments in Opelika, Lee County, AL, USA in 1999 after control failures with pyrethroid insecticides. This strain was subsequently selected with permethrin for several generations (Wei et al. 2001; Pridgeon et al. 2002), but not during this experiment. Wei et al. (2001) reported high levels of resistance in B. germanica to permethrin and deltamethrin, with resistance ratios of 97 and 480, respectively, compared with the susceptible strain. All cockroaches were reared at 25 ± 2 °C and 50 ± 10% RH with a photoperiod of 12L:12D. Dry dog chow and water were supplied ad libidum. One-week-old adult males from each strain were selected randomly and weighed on an electronic balance to the nearest 0.01 mg. Care was taken to avoid unnecessary stress to the cockroaches by allowing them to crawl individually into weighing vials and respirometers constructed from 3 ml syringes (Becton, Dickinson and Company, Ruthford, NJ, USA). Respirometers containing insects were connected to a manifold that provided dry, CO2-free air at the rate of 100 ml/min for ~ 10 min (Vogt and Appel, 1999). After flushing with dry CO2-free air, the plunger was brought to the 2 ml gradation and the stopcock was adjusted to seal the respirometer. Respirometers were incubated in the presence of a video camera at 5, 10, 15, 20, 25, 30, 35, and 40 ± 2 °C for 30 min. Temperature was checked with a calibrated mercury thermometer. Incubation took place under light from a 4 W red light bulb positioned approximately 50 cm above the experimental animals. The low level and color of the light facilitated filming of cockroaches during incubation by using a Panasonic GR-AX70u Compact VHS camera mounted on a tripod directly above the syringes containing cockroaches. Oxygen depletion and carbon dioxide enrichment in each respirometer was determined by using a Sable Systems International TR-3 respirometry system (Sable Systems International, Henderson, NV, USA). Outside air was scrubbed of CO2 and H2O by using a Whatman purge gas Generator (Whatman, Inc., Haverhill, MA, USA), drawn through a computer-controlled base lining system, a Li–Cor CO2 and H2O analyzer (LI-6262; LiCor Inc., Lincoln, Nebraska, USA), a Sable Systems FC-1 Oxygen Analyzer, and a Side-Track mass flow meter (Sierra Instruments Inc., Monterey, CA, USA) with a pump (GastMfg. Corp., Benton Harbor, MI, USA) at a rate of 100 ml/min at STP. The CO2 analyzer was calibrated with 94.9 ppm span gas (Air Products, Inc.). The oxygen analyzer was internally zeroed and spanned to 20.94% O2. From the 2 ml of air in a respirometer, 0.5 ml was injected into a glass T- injector port with a replaceable rubber septum and crimp seal installed upstream from the CO 2 -H2O analyzer. The gas sample passed through the system and data from the O2 and CO2 analyzers were recorded using DATACAN V (Version 5.2; Sable Systems International, Henderson, NV, USA) software. In one day, we tested groups of 5 male B. germanica of each strain at each temperature. This procedure was repeated four times (blocks) for a total of 20 insects per strain per temperature. Depletion (O2) and enrichment (CO2) volumes were integrated with respect to time. Rates of O2 consumption and CO2 production were calculated as the volume (ml) of O2 or CO2divided by insect body mass (g), and incubation time (min) resulting in VCO2 and VO2 (ml g-1 h-1). Respiratory quotient (RQ) was calculated as the ratio of CO2 produced (VCO2) to O2 consumed (VO2).
Supercooling points of six males of each strain were determined by using methods similar to those of Jones et al. (2008). Briefly, individual cockroaches were maintained at rearing conditions for 24 h without food, but with access to water. They were placed head down into 1 ml plastic micro-centrifuge tubes and held in place with a small piece of cotton. A copper-constantan thermocouple was threaded through the lid of the micro-centrifuge tube and positioned against the abdomen of the cockroach; the cotton held the thermocouple in place. Four tubes with cockroaches and thermocouples were inserted into a block of Styrofoam and placed into a freezer. The thermocouple wires were threaded through the gasket of freezer door and connected to a Sable Systems International TC-1000 thermocouple meter (Sable Systems International, Henderson, NV, USA). Temperature data were recorded at 0.5 sec intervals by using a laptop computer. Data were recorded until the exothermic (a spike in otherwise declining temperature) corresponding supercooling point was recorded.
Data were analyzed with the Proc Mixed procedure using SAS software (Littell et al. 1996) to determine the effects of cockroach strain on mean body mass, and to determine effects of temperature, strain, and the interaction of temperature and strain on VO2(ml g-1 h-1) and RQ. To elucidate possible interactions between the effects of temperature, cockroach strain, and mass on VO2 (ml g-1 h-1), an additional analysis was conducted using all possible combinations of main effects and interactions. The random effects of block and the block by strain interactions were included in all analyses. Models were reduced based upon examination of F-statistics for the main effects and interactions. Mean separation was carried out by using LS means. Linear regressions were performed where appropriate. Results are presented as means ± SE and a significance level of 0.05 was used throughout.
Results
Mean body mass of adult male cockroaches differed significantly among strains (F = 30.0, df = 2, 8, P = 0.0002): 0.0508 ± 0.0006, 0.0480 ± 0.0004, and 0.0543 ± 0.0005g for the black, resistant, and ACY strains, respectively. The overall effect of strain on VO2 (ml g-1 h-1) was not significant (P > 0.05), however different strains reacted differently to increasing temperature (F = 3.4, df = 16, 161, P < 0.0001). Temperature effects were examined by regressing log10-transformed mass-specific VO2 on temperature for each strain. For the black, ACY, and resistant strains, the relationship yielded the equations shown in Table 1.
The effect of temperature on oxygen consumption of the three strains (black, resistant, and ACY) of B. germanica is presented graphically in Fig.1. Q10 was calculated by multiplying the slope of the first-order log-linear regression of VO2 (ml g-1 h-1) on temperature by 10 and then taking the antilogarithm. This yields a mean Q10 through out the experimental temperature range of 1.77, 1.19, and 1.15 for the black, resistant and ACY strains, respectively. We fit a cubic equation to the temperature means (shown as solid lines in Fig. 1) to quantify changes in Q10 by differentiation of the polynomial equation (Lighton, 1989). The equations for the black, ACY, and resistant strains are shown in Table 2.
Table 1. Equations showing the relationship of log10-transformed mass-specific VO2on temperature for pyrethroid resistant and susceptible strains of B. germanica.

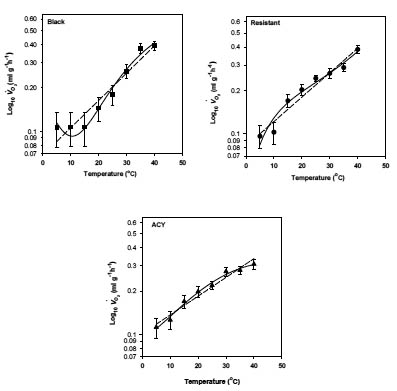
Fig. 1. Rate of oxygen consumption in pyrethroid resistant and susceptible strains of B. germanica at several temperatures. Broken line represents the first-order regression of log transformed oxygen consumption (ml g-1 h-1 ) on temperature, solid lines represents the third-order regression. See tables for equations.
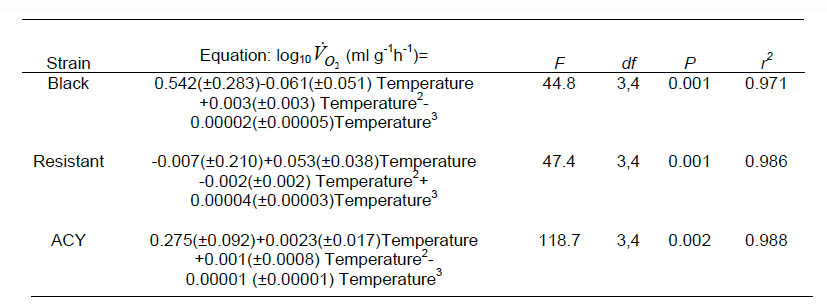
Table 2. Equations to quantify changes in Q10 in pyrethroid resistant and susceptible strains of B. germanica.
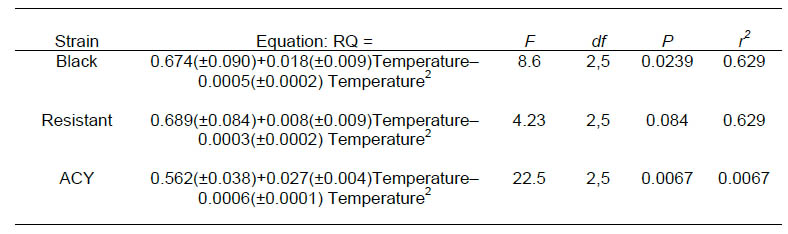
Strain alone did not explain a significant proportion of the variability in RQ (P>0.05) but the relationship between RQ and temperature differed among strains (F = 2.8, df = 16, 53, P = 0.0025). Estimated least square mean RQ for each strain were regressed over temperature. Respiratory quotient generally increased with temperature up to about 25 °C, declining thereafter in all the strains (Fig. 2). RQ at 25 °C and 15 °C for the ACY and resistant strains were highly variable between blocks and were considered outliers for this analysis. The equation relating RQ to temperature in the black, ACY and resistant strains are shown in Table 3.
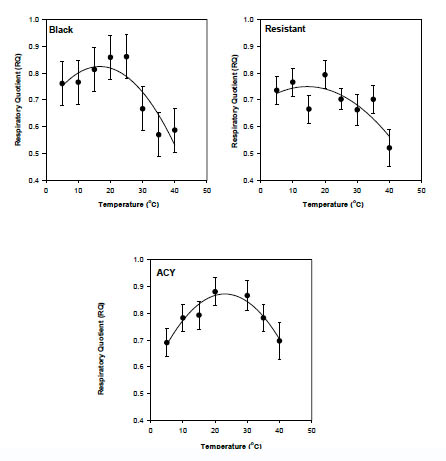
Fig 2. Relationship between temperature and RQ in pyrethroid resistant and susceptible strains of B. germanica.
Table 3. Equations relating RQ to temperature in pyrethroid resistant and susceptible strains of B. germanica.
All possible effects and interactions were included in a final model to determine their effect on raw VO2 (ml g-1 h-1). The reduced model included the effects of temperature (F = 73.1, df= 7, 357, P < 0.0001), mass (F = 12.1, df= 1, 364, P = 0.0006) and temperature by strain (F = 2.97, df = 16, 185, P = 0.0002). Since mass did not interact with the other main effects in the model, reduced models using mass-corrected VO2 are sufficient for predicting effects of temperature and strain on oxygen consumption. We used data at 25 °C to examine the effect of mass on the rate of oxygen consumption. The resulting relationship between VO2 (ml g-1h-1) and mass for the black, ACY and resistant strains are shown in Table 4 and in Fig. 3.
Table 4. The effects of mass on oxygen consumption in pyrethroid resistant and susceptible strains of B. germanica at 25o C.

The supercooling point of the resistant strain (-9.71 ± 0.47 °C) was significantly greater than the ACY (-11.36 ± 0.69 °C) and black (-11.67 ± 0.46 °C) susceptible strains.
Discussion
Insecticide resistance declines in the absence of selection in insects including, Bacillus thuringiensis resistant diamondback moth, Plutella xylostella (Tabashnik et al. 1994), and Colorado potato beetle, Leptinotarsa decemlineata (Rahardja and Whalon, 1995). Also, in pyrethroid resistant B. germanica, resistance levels declined from 140- to 1.6-fold in the absence of selection pressure after 15 generations (Cochran, 1993). This reversion is most probably associated with increased fitness (Tabashnik et al. 1994). For example, resistant beet armyworm pupae, Spodoptera exigua whose larvae were not exposed to Cry1C B. thuringiensis had similar metabolic rates as the susceptible strain whereas; pupae whose larvae were exposed continuously to toxin had greater metabolic rates (Dingha et al. 2004). Similarly, in this study, the metabolic rate of pyrethroid resistant B. germanica not exposed to insecticides for several generations was not significantly different from the susceptible stains. Hostetler and Brenner (1994), using closed system respirometry at 26 °C, and Dingha et al. (2005) using flow through respirometry at 10 °C, reported no significant difference in VO2 between pyrethroid resistant and susceptible B. germanica. By using microcalorimetry, Nielson et al. (2006) found no difference in metabolic heat production between chlorpyrifos resistant and susceptible strains of B. germanica that were not exposed to insecticide. However, heat production increased dramatically in the resistant strain in response to exposure to chlorpyrifos; there was no increase in heat production following insecticide exposure in the insecticide susceptible strains (Nielson et al. 2006). An explanation for the similarity in metabolic rate could be that the resistant strain of B. germanica may have detoxification mechanisms that would increase metabolic rates, but require the presence of insecticide to induce production of the detoxifying enzymes (Terriere, 1983).
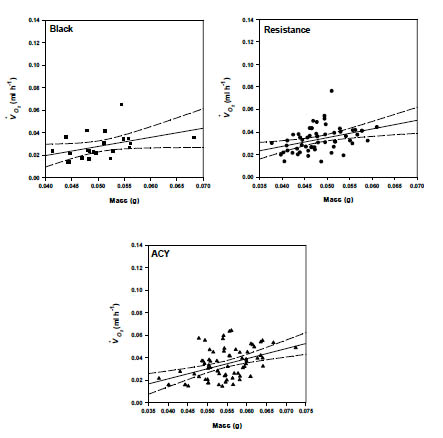
Fig 3. Mass scaling of rate of oxygen consumption (ml h-1) (adjusted to 25°C) of phyrethroid resistant and susceptible strains of B. germanica.
Even though metabolic rate was not significantly different among the strains, the three strains reacted differently to increasing temperatures. Generally, metabolic rate increases with increasing temperature however; exceptions from the exponential model have been reported in other insects (Keister and Buck, 1973) and fish (Jobling, 1994). In this study, the black strain is an exception; at 5, 10, and 15 °C, temperature had almost no effect on metabolism and the curve flattened (Fig. 1a). This may be due to reduced metabolic activities within this temperature range. At high temperatures of ~35 °C, the respiration rate in all three strains appeared to decrease (Fig. 1), indicating that 35 °C may be a physiologically stressful temperature.
The relationship between metabolism of an ectotherm and ambient temperature is best described by an exponential function (Q10) that depicts the magnitude of change in metabolic rate for a 10 °C change in temperature. Schmidt-Nielsen (1995), provides a great deal of information on the Q10 of the metabolic rate in insects, with values generally ranging from 1.5-3 (Prestwich and Walker, 1981; Davis et al. 1999; Rourke, 2000; Rogowitz and Chappell, 2000). The Q10 over the full temperature range (5–40 °C) for the three strains of B. germanica was 1.15 for the ACY, 1.19 for the resistant, and 1.77 for the black. At lower temperatures (5–15 °C) the Q10 was 1.00, 1.14, and 1.18 for the black, ACY and resistant strains, respectively. At higher temperature (20–35 °C), the Q10 was 1.20, 1.13, and 1.38 for the resistant, ACY and black strains, respectively. These values do not fall within the range predicted for insects. Q10 is predicted to be higher at lower temperatures (Schmidt-Nielsen, 1997), but this pattern is quite variable in insects. For example, the Q10 for a grasshopper did not change with temperature (Harrison and Fewell, 1995). Q10 did not vary with temperature in the resistant and ACY strains whereas in the black strain Q10 increased with temperature. Similarly, Q10 did not vary with temperature in two species of desert cockroach, Arenivaga apacha and A. investigata (Cohen and Cohen, 1981). The black strain may be better adapted to hotter environments and could have a higher temperature tolerance than the other two strains.
To estimate if there were differences in temperature tolerances among strains, we determined the supercooling point of each strain of B. germanica. The supercooling point is the temperature at which ice crystals form within the test organism (Leather et al. 1993). Presence of food in the midgut, existence of ice nucleating agents in the hemolymph, and contact with surface moisture could affect the supercooling points (Srmme, 1982). However, after maintaining adult males at rearing conditions without food (but with access to water) for 24 h, our preliminary results showed that the supercooling point of the resistant strain was significantly different than the ACY and black susceptible strains. The mechanisms of pyrethroid resistance in this strain of B. germanica include greater levels of P450 monooxygenases, hydrolases, and altered sodium channels (Pridgeon et al. 2002). It is possible that elevated expression of these enzymes by the resistant strain resulted in a greater supercooling point.
Rate of oxygen consumption increased with body mass in B. germanica (Fig. 3). The allometric exponent for the relationship between oxygen consumption and body mass was 0.78 for the resistant strain, 0.81 for the black strain and 0.89 for the ACY strain. VO2 in insects has previously been shown to scale with exponents between 0.6 and 1.0 (Mispagel, 1981; Vogt and Appel, 1999). The relationship between metabolic rate and body mass has been reported in other cockroaches for example, Blaberus discoidalis, where the scaling exponent was 0.83 (Birchard and Arendse, 2001). Among several cockroach species, including Periplaneta americana, Blatta orientalis, Leucophaea maderae the scaling exponent was 0.776 (Coelho and Moore, 1989). Gunn (1935) reported a scaling exponent of 0.7–0.8 for P. americana, B. orientalis, and B. germanica. Therefore, our findings are similar to those of other cockroaches. Although our values for cockroaches fall within the typical range, the mass scaling exponent varies considerably among and within the Insecta. In Diptera, VO2 scale with a mass exponent of 1 (Kittel, 1941), 0.407–1.28 in Coleoptera (Kittel, 1941; Bartholomew and Casey, 1977, May et al., 1986), 0.775 in Sphingidae and 0.814 in Saturniidae both Lepidoptera (Bartholomew, 1977). In contrast, the mass scaling values among vertebrates are similar (Coelho and Moore, 1989). The variability among insects could be explained by the fundamental difference between vertebrates and insects. The difference in the ratio of metabolically active to inactive tissues and organs may contribute to some of the observed differences.
Although RQ is not a proof of the identity of a particular substrate used in respiration, it nevertheless allows assumptions about the substrate metabolized (Withers, 1992). Generally, RQ values range from 0.71 for lipid, 0.80 for protein, and 1.00 for carbohydrate metabolism (Bartholomew, 1977). RQ values of B. germanica suggest that lipid and protein are the metabolic substrates. However, RQ values in B. germanica were affected by temperature indicating that the ratio of metabolic substrates changes with temperature. The RQ of all strains was greatest at the preferred temperatures of 20–30 °C. Lower or higher temperatures resulted in lower RQ values indicative of lipid metabolism. It is possible that lipid metabolism is more efficient or carbohydrate metabolism much less efficient at suboptimal temperatures. The insecticide susceptible strain had the most symmetrical response of RQ to temperature with the maximal RQ of ca. 0.875 at the colony rearing temperature of 25 °C (Fig. 2 ACY). The black and resistant strains had similar responses of RQ with temperature; RQ values at higher temperatures were much lower than at low temperatures. This effect may be a result of previous exposure of the black and resistant strains to insecticides, or simple differences in metabolic substrate use among strains.
In conclusion, VO2 (ml g-1 h-1) of B. germanica was not significantly different among strains, but different strains reacted differently to increasing temperature. The effects of temperature on the metabolic rate and RQ of pyrethroid resistant and susceptible B. germanica indicates that the fitness of different strains may be affected by temperature.
References
Angilletta, M. J., P. H. Niewiarowski and C. A. Navas. 2002. The evolution of thermal physiology in ectotherms. J. Thermal Biol. 27: 214-231.
Bartholomew, G. A. 1977. Energy metabolism, pp. 57-110. M. S. Gordon, G. A. Bartholomew, A. D. Grinnell, C. B. Jorgensen and F. N. White [Eds.], Animal physiology: Principles and adaptations. Macmillan publishing Co., Inc., New York.
Bartholomew, G. A. and T. M. Casey. 1977. Body temperature and oxygen consumption during rest and terrestrial activity in relation to body size in some tropical beetles. J. Therm. Biol. 2:173-176.
Birchard, G. F. and A. U. Arendse. 2001. An allometric analysis of oxygen consumption rate and cardiovascular function in the cockroach, Blaberus discoidalis. Comp. Biochem. Physiol. 129A: 339-344.
Cochran, D. G. 1989. Monitoring for insecticide resistance in field collected strains of the German cockroach (Dictyoptera:Blattellidae). J. Econ. Entomol. 82: 336-341.
Cochran, D. G. 1993. Decline of pyrethroid resistance in the absence of selection pressure in a population of German cockroaches (Dictyoptera: Blattelidae). J. Econ. Entomol. 86: 1639-1644.
Coelho, J. R. and A. J. Moore. 1989. Allometry of resting metabolic rate in cockroaches. Comp. Biochem. Physiol. 94A: 587-590.
Cohen, A. C. and J. L. Cohen. 1981. Microclimate, temperature and water relations of two species of desert cockroaches. Comp. Biochem. Physiol. 69A: 165-167.
Cossins, P. and K. Bowler. 1987. Temperature biology of animals. Chapman and Hall, London.
Davis, A. L. V., S. L. Chown and C. H. Scholtz. 1999. Discontinuous gas exchange in Scarabelus dung beetles (Coleoptera: Scarabaeidae): mass-scaling and temperature dependence. Physiol. Biochem. Zool. 72: 555-565.
Dingha, B. N., A. G. Appel and M. D. Eubanks. 2005. Discontinuous carbon dioxide release in the German cockroach, Blattella germanica (Dictyoptera: Blattellidae), and its effect on respiratory transpiration. J. Insect Physiol. 51: 825-836.
Dingha, B. N., W. J. Moar and A. G. Appel. 2004. Effects of Bacillus thuringiensis Cry1C toxin on the metabolic rate of Cry1C resistant and susceptible Spodoptera exigua (Lepidoptera: Noctuidae). Physiol. Entomol. 29: 409-418.
Gillooly, J. F., J. H. Brown and G. B. West. 2001. Effects of size and temperature on metabolic rate. Sci. 21: 2248-2251.
Gunn, D. L. 1935. The temperature and humidity relations of the cockroach. III. A comparison of temperature preference, and rates of desiccation and respiration of Periplaneta americana, Blatta orientalis and Blattella germanica. J. Exp. Biol. 12: 185-190.
Harrison, J. F. and J. H.Fewell. 1995. Thermal effects on feeding behavior and net energy intake in a grasshopper experiencing large diurnal fluctuations in body temperatures. Physiol. Zool. 68: 453-473.
Hawkins, A. J. 1995. Effects of temperature change on ectotherm metabolism and evolution: Metabolic and physiological interrelations underlying the superiority of multi-locus heterozygotes in heterogeneous environments. J. Therm. Biol.20: 23-33.
Hostetler, M. E. and R. J. Brenner. 1994. Behavioral resistance to insecticide in the German cockroach (Dictyoptera: Blattellidae) an experimental reevaluation. J. Econ. Entomol. 87: 885-893.
Huey, R. B. and D. Berrigan. 2001. Temperature, demography, and ectotherm fitness. Am. Nat. 158: 204-210.
Jobling, M. 1994. Fish energetics. Chapman and Hall, London.
Jones, D. B., K. L. Giles and N. C. Elliott. 2008. Supercooling points of Lysiphlebus testsceipes and its host Schizaphis graminum. Environ. Entomol. 37: 1063-1068.
Keister, M. and J. Buck. 1973. Respiration: Some exogenous and endogenous effects on rate of respiration, pp.469-505. In: M. Rockstein [Ed.], The physiology of Insecta, Vol. V1. Academic Press, New York and London,.
Kittel A. 1941. Körpergröβe, Körperzeiten und Energiebilanz. II. Der Sauerstoffverbrauch der Insekten in Abhängigkeit von der Körpergröβe. Z. f. vergl. Physiol. 28: 533-562.
Kolluru, G. R., M. Zuk and M. A. Chappell. 2002. Reduced reproductive effort in male field crickets infested with parasitoid fly larvae. Behav. Ecol. 13: 607-614.
Kramarz, P. and A. Kafel. 2003. The respiration rate of the beet armyworm pupae (Spodoptera exigua) after multi-generation intoxication with cadmium and zinc. Environ. Pollution 126: 1-3.
Leather, S. R., K. F. A. Walters and J. S. Bale. 1993. The ecology of insect overwintering. Cambridge University Press, Cambridge.
Lighton, J. R. B. 1989. Individual and whole-colony respiration in an African formicine ant. Functional Ecology 3: 523-530.
Littell, R.C., Milliken, G.A., Stroup, W.W. and Wolfinger, R.D. 1996. SAS system for mixed models. SAS Institute Inc., Cary, N.C.
May, M. L., D.L. Pearson, and T.M. Casey. 1986. Oxygen consumption of active and inactive tiger beetles. Physiol Entomol. 11: 171-179.
Mispagel, M. E. 1981. Relation of oxygen consumption to size and temperature in desert arthropods. Ecol. Entomol. 6: 423-432.
Nielsen, M. G., G. W. Elmes and V. E. Kipyatkov. 1999. Respiratory Q10 varies between populations of two species of Myrmica ants according to the latitude of their sites. J. Insect. Physiol. 45: 559-564.
Nielsen, S. A., K.-M. Vagn Jensen, M. Kristensen and P. Westh. 2006. Energetic cost of subacute Chlorpyrifos intoxication in the German cockroach (Dictyoptera: Blattellidae). Environ. Entomol. 35: 837-842.
Prestwich, K. N. and T. J. Walker. 1981. Energetics of singing in crickets: effect of temperature in three trilling species (Orthoptera: Gryllidae). Oecologia (Berlin) 143: 199-212.
Pridgeon, J. W., Appel, A. G., Moar, W. J., Liu, N., 2002. Variability of resistance mechanisms in pyrethroid resistanct German cockroaches (Dictypotera: Blattellidae). Pestic. Biochem. Physiol. 73: 149-156.
Rahardja, U. and M. E. Whalon. 1995. Inheritance of resistance to Bacillus thuringiensis subsp. tenebrionis CryIII A delta-endotoxin in Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 88: 21-26.
Ramirez, P. J. 1989. The cockroach as a vector of pathogenic agents. Boletin de la Oficina Sanitaria Panamericana 107: 41-53.
Roberts, J. 1996. Cockroaches linked with asthma. British Medical Journal 312: 1630.
Rogowitz, G. L. and M. A. Chappell. 2000. Energy metabolism of eucalyptus-boring beetles at rest and during locomotion: gender makes a difference. J. Exp. Biol. 203: 1131-1139.
Ross, M. H. and D. G. Cochran. 1975. The German cockroach, Blattella germanica, pp. 35-62. In: R. C. King [Ed.], Invertebrates of genetic interest. Plenum Press, New York.
Rourke, B. 2000. Geographic and altitudinal variation in water balance and metabolic rate in the California grasshopper, Melanoplus sanguinipes. J. Exp. Biol. 203: 2699-2712.
Schmidt-Nielsen, K. 1995. Animal physiology. Cambridge University Press, New York.
Srmme, L. 1982. Supercooling and winter survival in terrestrial arthropods. Comp. Biochem. Physiol. 73A: 519-543.
Tabashnik, B. E., N. Finson, F. R. Groeters, W. J. Moar, M. W. Johnson, K. Luo and M. J. Adang. 1994. Reversal of resistance to Bacillus thuringiensis in Plutella zylostella. Proc. Nat. Acad. Sci. 91: 4120-414.
Terriere, L.C. 1983. Enzyme induction, gene amplification and insect resistance to insecticides, pp. 265-298. In: R. T. Roush and B. E. Tabashnik [Eds.], Pesticide resistance in arthropods. Chapman and Hall, New York.
Valles, S. M., K. Dong and R. J. Brenner. 2000. Mechanisms responsible for cypermethrin resistance in a strain of German cockroach, Blattella germanica.Pestic. Biochem. Physiol. 66: 195-205.
Vogt, J. T. and A. G. Appel. 1999. Standard metabolic rate of the fire ant, Solenopsis invicta Buren: effects of temperature, mass and caste. J. Insect Physiol. 45: 655-666.
Wei, Y., A. G. Appel, W. J. Moar and N. Liu. 2001. Pyrethroid resistance and cross-resistance in the German cockroach, Blattella germanica (L.). Pest. Manag. Sci. 57: 1055-1059.
Withers, P. C. 1992. Comparative animal physiology. Saunders, New York.
