Report
The Discovery and Pursuit of American Boutonneuse Fever: A New Spotted Fever Group Rickettsiosis [pdf]
J. Goddard1 and A. Varela-Stokes2
1Department of Entomology and Plant Pathology, Mississippi State University
2Department of Basic Sciences, College of Veterinary Medicine, Mississippi State University
Accepted: 1-XII-2008
Abstract. There is a new tick-borne disease similar to Rocky Mountain spotted fever (RMSF) that was recently described from the southern United States. Cases of the disease are a subset of reported RMSF cases and therefore constitute a “disease within a disease.” The causative agent of this RMSF-like disease, however, is Rickettsia parkeri as opposed to R. rickettsii, the pathogen causing RMSF. Both organisms are in the spotted fever group (SFG) of rickettsiae, cross-react in laboratory tests used to diagnose RMSF, and therefore are difficult to differentiate serologically. The new malady has been tentatively named “American Boutonneuse Fever” (ABF) based on clinical similarities with Boutonneuse Fever, a spotted fever-like disease also known as Mediterranean spotted fever, which occurs in the Eastern Hemisphere. This paper highlights several research projects currently underway at Mississippi State University to investigate the prevalence, epidemiology, and natural history of ABF, as well the ecology of its tick vector.
History and Background
Rickettsia parkeri, at the time just called the “maculatum agent,”was first isolated in 1937 from Amblyomma maculatum (Gulf Coast tick; GCT, Figure 1) collected from cattle in Texas (Parker et al. 1939). The organism (Figure 2) was considered a non-pathogenic rickettsia despite some initial speculation that it might cause human disease. Later, the organism was named R. parkeri in honor of Dr. Parker (Lackman et al., 1965). Interestingly, Dr. Parker noted how this bacterium resembled R. conorii, the agent of boutonneuse fever (Mediterranean spotted fever) and R. africae, the causative agent of African tick-bite fever (Paddock et al., 2004). Eventually, it was discovered that these rickettsial species are genetically related (Fournier et al., 1998; Roux and Raoult, 2000). The genetic relationships provided further evidence that R. parkeri may be pathogenic to humans as well. In the 1950s, Philip and White found evidence of R. parkeri in GCT from Mississippi, and the agent has been identified in GCT collected in many states within the tick’s range (Figure 3) (Philip and White, 1955; Sumner et al., 2007). In addition, in the 1980s, Goddard and Norment identified R. parkeri in field collections of the Lone Star tick (LST), Amblyomma americanum in Mississippi (Goddard and Norment, 1986). This was a new finding that prompted the senior author to experimentally infect a laboratory colony of LST with the agent. Extensive experiments with that infected colony revealed that LST could transstadially and transovarially transmit R. parkeri through at least two generations (Goddard, 2003). In addition, notes made during the feeding of infected ticks on guinea pigs indicated that the agent could produce mild fever, scrotal reactions, and also spots of necrosis, known as eschars, at the sites of tick attachment. Interestingly, R. rickettsii, the causative agent of Rocky Mountain spotted fever (RMSF) does not cause these eschars.
Figure 1. Adult female Gulf Coast tick, Amblyomma maculatum (Photo courtesy Dr. Blake Layton, Mississippi State University Extension Service).
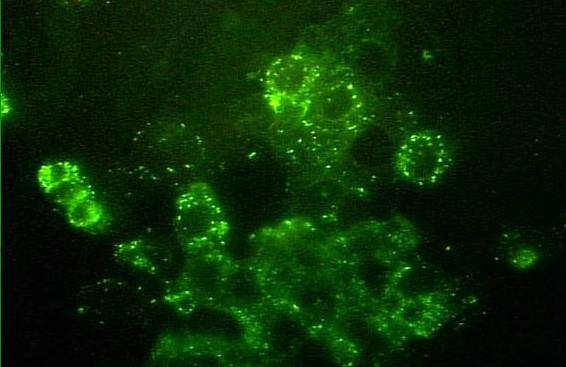
Figure 2. Rickettsia parkeri in cell culture (fluorescent antibody staining).
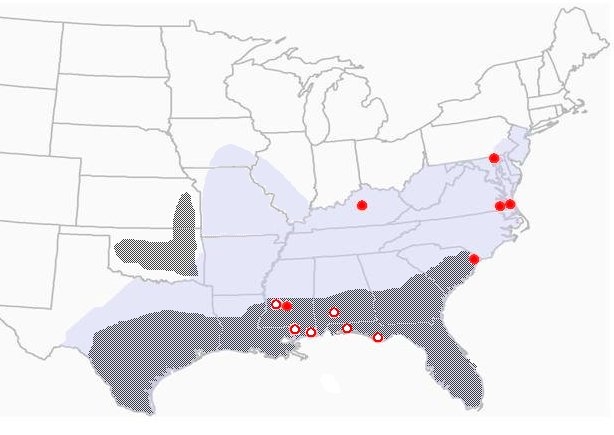
Figure 3. Geographic distribution of the Gulf Coast tick showing presumed and confirmed human cases of American boutonneuse fever (ABF). Solid circles are confirmed cases; darker shading represents established/primary distribution of the tick (Drawing courtesy Dr. Kristine T. Edwards, Mississippi State University).
The first report of human infection with R. parkeri occurred in 2002, when Dr. Chris Paddock, a CDC researcher, identified the agent in a case of spotted fever-like illness in a 40-year-old man from the coast of Virginia (Paddock et al., 2004). The man complained of fever, headache, malaise, myalgias, arthralgias, and (most notably) spots of necrosis (eschars) on his legs where the ticks had presumably fed. Other reports of human disease caused by this organism prompted a re-evaluation of its nonpathogenic status (Paddock, 2005). In 2006, a 53-year-old man who had visited the Virginia Beach area presented with a flu-like illness characterized by fever, malaise, and a rash. He reportedly removed a tick, which was described as most likely a GCT, and had developed an eschar at the site of the tick bite (Whitman et al., 2007). In 2006, Finley et al. (2006) presented a case of R. parkeri infection causing clinical symptoms in a human patient from Mississippi. Since then, several more cases of R. parkeri causing disease in humans have been observed and reported (Figure 3) (Paddock et al., 2008).
Although there is no published convention for naming disease entities (as opposed to naming zoological organisms), names are generally placed on diseases as useful labels. “Maculatum infection” was used by Parker to describe the disease originally, however, since then, a more fitting name has been proposed. In 2004, Dr. Goddard noted the similarity between American cases of R. parkeri infection and “boutonneuse fever,” which occurs in Europe and Africa. As mentioned, there is evidence for a genetic relationship between R. parkeri and R. conorii. In guinea pig experiments, both agents caused eschars at the bite site similar to those reported in both humans and animals (JG, unpublished observations). Based on these similarities, it was proposed that R. parkeri human infection be named “American Boutonneuse Fever” (ABF) (Goddard, 2004).
Current research on American Boutonneuse fever
For several reasons, Mississippi is uniquely positioned for further studies to establish the risk of human infection with R. parkeri, as well as studies on the natural history of the organism. First of all, Mississippi is the state where R. parkeri was first identified in the LST, and secondly, infection rates of GCTs with R. parkeri are apparently higher there than in other states, leading to more human cases than any other state (see Figure 3). Infection rates of field-caught GCTs with R. parkeri have ranged from approximately 10% in Florida to 17% in Georgia (Sumner et al. 2007). Preliminary work during 2007, however, revealed infection rates as high as 40% in GCTs along the Mississippi Gulf Coast (CDC unpublished data).Our efforts to evaluate human risk for infection will benefit both the public and health professionals as they become more aware of these emerging diseases. Studies on the natural history of R. parkeri may help public health officials and pest control personnel know how to intervene, thus slowing or preventing disease transmission.
Increased surveillance for cases of ABF. One of the first things the authors did after the discovery of the new disease was to increase statewide surveillance for new cases. This involved help from personnel at the Epidemiology Division of the Mississippi Department of Health (MDH) who have a statewide network of health department clinics, nine district offices, and at least nine “epidemiology nurses” whose job is specifically to locate cases of diseases of public health importance. An article was placed in the statewide MDH publication, Mississippi Morbidity Report, asking physicians to be on alert for cases of ABF. In addition, MDH has helped arrange continuing medical education events in the southern parts of the state to inform local doctors of the new disease. More CME events are planned.
Role of cattle in the natural history of R. parkeri. Although it has been 70 years since the first isolation of R. parkeri, little has been done to try to determine the natural history of this organism, likely due to its original non-pathogenic status. We do not know what wildlife maintains the organism, nor its prevalence in ticks and vertebrate animals. Our research goals are to understand the maintenance of this organism in nature. Cattle are a good host for adult stages of GCT and experiments have been conducted by the authors and a graduate student, Kristine Edwards DVM, MPH, at Mississippi State University Veterinary School, to determine their possible role in the natural history of R. parkeri.
One of our first objectives for evaluating cattle as a host for the pathogen involved experimental studies using Holstein calves. In these studies, cattle were experimentally exposed to R. parkeri,either via needle inoculation with the cultivated organism or via infestation of cattle with R. parkeri infected adult A. maculatum ticks. Control calves that were not exposed to R. parkeri but received a similar treatment were included in the study. There were no changes in hematology or blood chemistry in any calves exposed to R. parkeri that indicated rickettsial disease. Those calves did seroconvert, however, and several exposed calves were transiently positive for circulating organism by PCR analysis of blood. In addition to the experimental studies, we have been interested in evaluating cattle for evidence of natural exposure to the organism. To date, samples of whole blood and serum have been collected from nearly 200 cattle located in six sale barns within Mississippi. Biopsies of the ears have also been collected from cattle infested with ticks. No evidence of R. parkeri DNA has been detected in the cattle tested thus far by PCR. Visual evidence of rickettsiae has been found in the hemolymph of some ticks, by Gimenez staining, but will be analyzed further to determine the specific identity of the organism.
Role of wildlife in the natural history of R. parkeri. Birds and wild rodents, including cotton rats, are hosts for immature stages of the GCT and may serve as reservoirs for infecting new ticks with R. parkeri. Larger mammals, including cattle, sheep and some wildlife, are hosts for the adult stages. Experiments are underway by two other MSU graduate students, Ashley Harris and Gail Moreau, to assess the role of these vertebrates in the life cycle of ABF. While Ashley has focused on assessing larger mammals such as white-tailed deer and feral swine for evidence of R. parkeri infection, Gail is focusing on rodents and birds.
Because our laboratory is also interested in other tick-borne diseases, samples from deer, feral swine, raccoons and opossums have also been evaluated for evidence of infection with Ehrlichia chaffeensis (the agent of human monocytic ehrlichiosis) and Borrelia lonestari (the putative agent of “southern tick-associated rash illness” or STARI) as well as R. parkeri. White-tailed deer had the highest evidence of infection or exposure to E. chaffeensis and B. lonestari while raccoons had the highest evidence of exposure to R. parkeri. Ashley is continuing this work as part of her Master’s degree.
Gail is pursuing both a PhD and a DVM in a dual-degree program. She will be conducting experiments to evaluate host preferences for the immature stages of GCT and use field evidence of infection in wild rodents and birds to determine the reservoir host potential of the organism. We hypothesize that immature ticks play a large role in the maintenance of this organism and the hosts they feed on may serve as important reservoirs for R. parkeri.
Ecological studies with the GCT, vector of ABF. Tick-disease-host relationships are complex and affected by weather, day length and other ecological factors. Accordingly, studies on tick ecology, and their related pathogens, are much needed. Previous ecological studies pertinent to the GCT include the effect of weather on questing populations of hard ticks (Goddard, 1992, 2001) as well as mark-release-recapture experiments to estimate tick populations in Mississippi forests (Goddard, 1993; Goddard and Goddard, 2008). Studies on the spatial and geographic distribution of a related tick, A. americanum, revealed that they “cluster” in spots in the woods, and are not evenly distributed (Goddard, 1997). For example, the majority of adult and nymphal ticks were collected in only 17.7% and 9.7% of the field plots, respectively. In addition, ticks, being subject to desiccation, are found in predominantly shady spots. Goddard (1997) found 21/31 (68%) of adult ticks and 24/33 (73%) of nymphs were collected in areas of 71% and 65% shade, respectively.
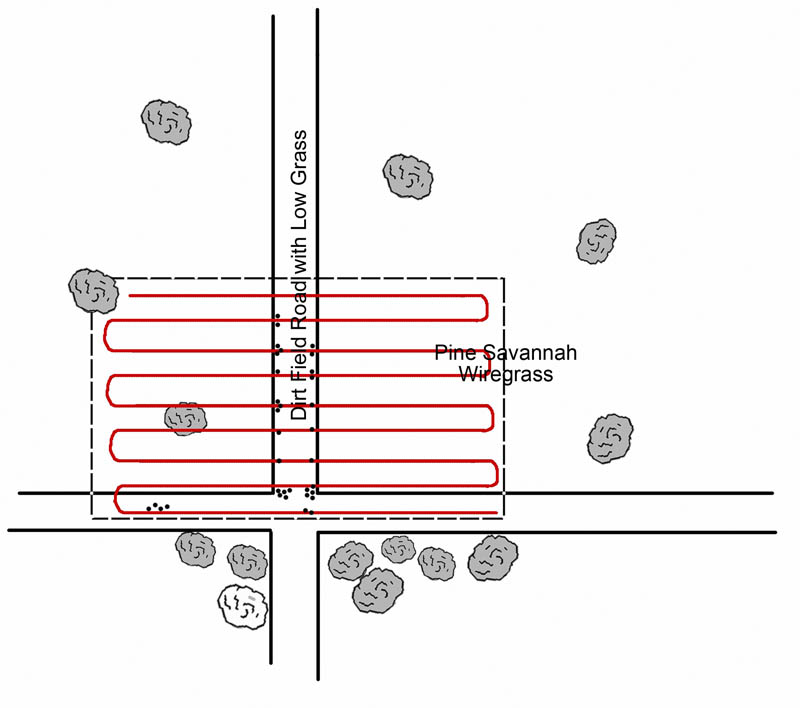
Oddly, preliminary data with the Gulf Coast tick reveals just the opposite behavior. Careful collections of the tick along the Mississippi Gulf Coast have shown that adults of this tick are found in open sunshine, with little relation to shade or soil moisture. One 0.5-ha collection site within the Grand Bay National Wildlife Refuge yielded over 100 adult A. maculatum (Figure 4). Systematic collections by using a drag cloth within the Sandhill Crane National Refuge revealed that most A. maculatum were found along the road—in bright sunshine—as opposed to in wooded areas (Figure 5, dots represent tick collections).
Summary
In addition to the future research planned by our laboratories, further ecological research is needed to determine the parameters dictating A. maculatum questing and host-finding activity, especially in relation to the new disease ABF and our changing climate. Knowledge about these parameters could theoretically lead to precision-targeting of pesticides or other pest control interventions to lower human exposure to these disease-bearing ticks.

Figure 4. Collection site of numerous Gulf Coast ticks, showing almost no shade.
Figure 5. Collections of Gulf Coast ticks in the Sandhill Crane National Wildlife Refuge. Red lines are collecting “paths;” dots represent ticks collected.
References
Finley, R. W., J. Goddard, D. Raoult, M. E. Eremeeva, R. D. Cox, C. D. Paddock. 2006. Rickettsia parkeri: a case of tick-borne, eschar-associated spotted fever in Mississippi. International Conference on Emerging Infectious Diseases, Abstract No. 188.
Fournier, P. E., V. Roux, D. Raoult. 1998. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol.48 Pt 3: 839-849.
Goddard, J. 1992. Ecological studies of adult Ixodes scapularis in central Mississippi: questing activity in relation to time of year, vegetation type, and meteorologic conditions. J. Med. Entomol.29: 501-506.
Goddard, J. 1993. Ecological studies of Ixodes scapularis (Acari: Ixodidae) in central Mississippi: lateral movement of adult ticks. J. Med. Entomol.30: 824-826.
Goddard, J. 1997. Clustering of lone star ticks: Implications for control. J Env Hlth59, 8-11.
Goddard, J. 2001. Ticks and tick ecology: Implications for human disease transmission. J. Mississippi Acad. Sci.46: 100-104.
Goddard, J. 2003. Experimental infection of lone star ticks, Amblyomma americanum (L.), with Rickettsia parkeri and exposure of guinea pigs to the agent. J. Med. Entomol.40: 686-689.
Goddard, J. 2004. American Boutonneuse Fever: a new tick-borne rickettsiosis. Inf. Med.21: 207-210.
Goddard, J. 2nd, J. Goddard. 2008. Estimating populations of adult Ixodes scapularis in Mississippi using a sequential Bayesian algorithm. J. Med. Entomol.45: 556-562.
Goddard, J., B. R. Norment. 1986. Spotted fever group rickettsiae in the lone star tick, Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol.23: 465-472.
Lackman, D. B., E.J . Bell, H. G. Stoenner, E. G. Pickens. 1965. The Rocky Mountain Spotted Fever Group of Rickettsias. Health Lab. Sci.2: 135-141.
Paddock, C. D., J. W. Sumner, J. A. Comer, S. R. Zaki, C. S. Goldsmith, J. Goddard, S. L. McLellan, C. L. Tamminga, C.A. Ohl. 2004. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis.38: 805-811.
Paddock, C. D. 2005. Rickettsia parkeri as a paradigm for multiple causes of tick-borne spotted fever in the western hemisphere. Ann. N. Y. Acad. Sci.1063: 315-326.
Paddock, C. D., R. W. Finley, C. S. Wright, H. N. Robinson, B. J. Schrodt, C. C. Lane, O. Ekenna, M. A. Blass, C. L. Tamminga, C. A. Ohl, S. L. McLellan, J. Goddard, R. C. Holman, J. J. Openshaw, J. W. Sumner, M.E. Eremeeva. 2008. Maculatum infection, a tick-transmitted disease caused by Rickettsia parkeri. Clin. Infect. Dis. 47: 1188-1196.
Parker, R. R., G. M. Kohls, G. W. Cox, G. E. Davis. 1939. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep.54: 1482-1484.
Philip, C. B., J. S. White. 1955. Disease agents recovered incidental to a tick survey of the Mississippi Gulf Coast. J. Econ. Entomol.48: 396-400.
Roux, V., D. Raoult. 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol.50 Pt 4: 1449-1455.
Sumner, J. W., L. A. Durden, J. Goddard, E. Y. Stromdahl, K. L. Clark, W. K. Reeves, C. D. Paddock. 2007. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg. Infect. Dis.13: 751-753.
Whitman, T. J., A. L. Richards, C. D. Paddock, C. L. Tamminga, P. J. Sniezek, J. Jiang, D. K. Byers, J. W. Sanders. 2007. Rickettsia parkeri infection after tick bite, Virginia. Emerg. Infect. Dis.13: 334-336.